174+ Neutral Nitrogen Atom
174+ Neutral Nitrogen Atom. It has an atomic weight of 14.007 amu. The average atomic mass of a nitrogen atom is 14. A neutral atom of nitrogen will have five valence electrons.
Nejchladnější Illustration Nitrogen Atom Stock Vector Royalty Free 1672488919
Hence, the given element has 7 protons, 7 electrons and 7 neutrons. I show you where nitrogen is on the periodic table and how to determine. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … This element is found in group 15 and period 2 of the periodic table of the elements.Atomic number = number of electrons = number of protons = 7.
The average atomic mass of a nitrogen atom is 14. The number of protons is the atomic number, which for nitrogen is 7. Nitrogen is the seventh element on the periodic table. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. This element is found in group 15 and period 2 of the periodic table of the elements. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons.
Atomic number = number of electrons = number of protons = 7. Nitrogen is the seventh element on the periodic table. I show you where nitrogen is on the periodic table and how to determine. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus.. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.

Atomic mass of nitrogen = 14.01 amu. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. Atomic number = number of electrons = number of protons = 7. I show you where nitrogen is on the periodic table and how to determine... This is the sum of protons and neutrons in the nucleus.

Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus... Hence, the given element has 7 protons, 7 electrons and 7 neutrons. Nitrogen is the seventh element on the periodic table. This is the sum of protons and neutrons in the nucleus. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. It has an atomic weight of 14.007 amu. Atomic mass of nitrogen = 14.01 amu. Neutral atoms will have the same number of protons in the nucleus as they have electrons. The average atomic mass of a nitrogen atom is 14. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. It has an atomic weight of 14.007 amu.

Atomic number = number of electrons = number of protons = 7. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. It has an atomic weight of 14.007 amu. A neutral atom of nitrogen will have five valence electrons. Nitrogen is the seventh element on the periodic table.. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.

A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge... The number of protons is the atomic number, which for nitrogen is 7. The number of protons is the atomic number, which for nitrogen is 7.

The average atomic mass of a nitrogen atom is 14. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. It has an atomic weight of 14.007 amu. Atomic number = number of electrons = number of protons = 7. I show you where nitrogen is on the periodic table and how to determine. I show you where nitrogen is on the periodic table and how to determine.

Atomic number = number of electrons = number of protons = 7... This is the sum of protons and neutrons in the nucleus. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. A neutral atom of nitrogen will have five valence electrons. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … I show you where nitrogen is on the periodic table and how to determine. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.

The number of protons is the atomic number, which for nitrogen is 7... The number of protons is the atomic number, which for nitrogen is 7. Atomic mass of nitrogen = 14.01 amu. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. This element is found in group 15 and period 2 of the periodic table of the elements. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. This is the sum of protons and neutrons in the nucleus. It has an atomic weight of 14.007 amu. A neutral atom of nitrogen will have five valence electrons. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons.

Atomic mass of nitrogen = 14.01 amu. Neutral atoms will have the same number of protons in the nucleus as they have electrons. Atomic number = number of electrons = number of protons = 7... In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in …

A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. This element is found in group 15 and period 2 of the periodic table of the elements. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … This is the sum of protons and neutrons in the nucleus. A neutral atom of nitrogen will have five valence electrons.

This is the sum of protons and neutrons in the nucleus. Neutral atoms will have the same number of protons in the nucleus as they have electrons. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge... In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in …

Neutral atoms will have the same number of protons in the nucleus as they have electrons. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Neutral atoms will have the same number of protons in the nucleus as they have electrons. I show you where nitrogen is on the periodic table and how to determine. The number of protons is the atomic number, which for nitrogen is 7.. Neutral atoms will have the same number of protons in the nucleus as they have electrons.

The number of protons is the atomic number, which for nitrogen is 7. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. This element is found in group 15 and period 2 of the periodic table of the elements. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. I show you where nitrogen is on the periodic table and how to determine... Atomic number = number of electrons = number of protons = 7.

In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … This is the sum of protons and neutrons in the nucleus. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. A neutral atom of nitrogen will have five valence electrons. This element is found in group 15 and period 2 of the periodic table of the elements. The number of protons is the atomic number, which for nitrogen is 7. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.

Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. The average atomic mass of a nitrogen atom is 14. Nitrogen is the seventh element on the periodic table. I show you where nitrogen is on the periodic table and how to determine. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. Atomic mass of nitrogen = 14.01 amu... Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.

Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Neutral atoms will have the same number of protons in the nucleus as they have electrons. The average atomic mass of a nitrogen atom is 14. A neutral atom of nitrogen will have five valence electrons. I show you where nitrogen is on the periodic table and how to determine. This element is found in group 15 and period 2 of the periodic table of the elements.

This is the sum of protons and neutrons in the nucleus.. Atomic number = number of electrons = number of protons = 7. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. Nitrogen is the seventh element on the periodic table. I show you where nitrogen is on the periodic table and how to determine.. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus.

A neutral atom of nitrogen will have five valence electrons... The average atomic mass of a nitrogen atom is 14. Nitrogen is the seventh element on the periodic table. Neutral atoms will have the same number of protons in the nucleus as they have electrons. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. Atomic number = number of electrons = number of protons = 7.. Neutral atoms will have the same number of protons in the nucleus as they have electrons.

It has an atomic weight of 14.007 amu. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. Neutral atoms will have the same number of protons in the nucleus as they have electrons. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. A neutral atom of nitrogen will have five valence electrons. Atomic mass of nitrogen = 14.01 amu. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. Nitrogen is the seventh element on the periodic table... Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons.

Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge... A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. It has an atomic weight of 14.007 amu. The average atomic mass of a nitrogen atom is 14. Atomic mass of nitrogen = 14.01 amu. Neutral atoms will have the same number of protons in the nucleus as they have electrons. Nitrogen is the seventh element on the periodic table. This element is found in group 15 and period 2 of the periodic table of the elements. The number of protons is the atomic number, which for nitrogen is 7.

The average atomic mass of a nitrogen atom is 14. It has an atomic weight of 14.007 amu. The average atomic mass of a nitrogen atom is 14. Atomic mass of nitrogen = 14.01 amu. The number of protons is the atomic number, which for nitrogen is 7. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. This element is found in group 15 and period 2 of the periodic table of the elements. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. I show you where nitrogen is on the periodic table and how to determine. This element is found in group 15 and period 2 of the periodic table of the elements.

Atomic number = number of electrons = number of protons = 7.. This is the sum of protons and neutrons in the nucleus. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. Nitrogen is the seventh element on the periodic table. It has an atomic weight of 14.007 amu.. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge.

The average atomic mass of a nitrogen atom is 14. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … It has an atomic weight of 14.007 amu. Atomic number = number of electrons = number of protons = 7. Atomic mass of nitrogen = 14.01 amu. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. The average atomic mass of a nitrogen atom is 14.

In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … . Hence, the given element has 7 protons, 7 electrons and 7 neutrons.

The average atomic mass of a nitrogen atom is 14... Atomic mass of nitrogen = 14.01 amu.. Atomic mass of nitrogen = 14.01 amu.

The average atomic mass of a nitrogen atom is 14. It has an atomic weight of 14.007 amu. This element is found in group 15 and period 2 of the periodic table of the elements. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … I show you where nitrogen is on the periodic table and how to determine. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. The average atomic mass of a nitrogen atom is 14. A neutral atom of nitrogen will have five valence electrons. Atomic mass of nitrogen = 14.01 amu. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. Neutral atoms will have the same number of protons in the nucleus as they have electrons.

Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. I show you where nitrogen is on the periodic table and how to determine. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons.. Hence, the given element has 7 protons, 7 electrons and 7 neutrons.

The average atomic mass of a nitrogen atom is 14.. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. Atomic number = number of electrons = number of protons = 7. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. It has an atomic weight of 14.007 amu. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. The number of protons is the atomic number, which for nitrogen is 7. This is the sum of protons and neutrons in the nucleus. Atomic mass of nitrogen = 14.01 amu. I show you where nitrogen is on the periodic table and how to determine. Nitrogen is the seventh element on the periodic table.

Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … I show you where nitrogen is on the periodic table and how to determine. Nitrogen is the seventh element on the periodic table. The average atomic mass of a nitrogen atom is 14. This is the sum of protons and neutrons in the nucleus. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. Atomic mass of nitrogen = 14.01 amu. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. It has an atomic weight of 14.007 amu.

Neutral atoms will have the same number of protons in the nucleus as they have electrons.. This element is found in group 15 and period 2 of the periodic table of the elements... In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in …

This element is found in group 15 and period 2 of the periodic table of the elements. Atomic mass of nitrogen = 14.01 amu. Nitrogen is the seventh element on the periodic table. Atomic number = number of electrons = number of protons = 7. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. A neutral atom of nitrogen will have five valence electrons.

This element is found in group 15 and period 2 of the periodic table of the elements. Atomic mass of nitrogen = 14.01 amu. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. The number of protons is the atomic number, which for nitrogen is 7. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. The average atomic mass of a nitrogen atom is 14. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … A neutral atom of nitrogen will have five valence electrons. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. Atomic number = number of electrons = number of protons = 7.

Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. A neutral atom of nitrogen will have five valence electrons. This element is found in group 15 and period 2 of the periodic table of the elements. I show you where nitrogen is on the periodic table and how to determine. The number of protons is the atomic number, which for nitrogen is 7. The average atomic mass of a nitrogen atom is 14. A neutral atom of nitrogen will have five valence electrons.

Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. The number of protons is the atomic number, which for nitrogen is 7. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. A neutral atom of nitrogen will have five valence electrons. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons.

Neutral atoms will have the same number of protons in the nucleus as they have electrons. This element is found in group 15 and period 2 of the periodic table of the elements. Neutral atoms will have the same number of protons in the nucleus as they have electrons. This is the sum of protons and neutrons in the nucleus. Atomic number = number of electrons = number of protons = 7. The number of protons is the atomic number, which for nitrogen is 7. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … Nitrogen is the seventh element on the periodic table. The average atomic mass of a nitrogen atom is 14.. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.

This element is found in group 15 and period 2 of the periodic table of the elements. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. The average atomic mass of a nitrogen atom is 14.. Neutral atoms will have the same number of protons in the nucleus as they have electrons.

This element is found in group 15 and period 2 of the periodic table of the elements. It has an atomic weight of 14.007 amu. The average atomic mass of a nitrogen atom is 14. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. Neutral atoms will have the same number of protons in the nucleus as they have electrons. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. The number of protons is the atomic number, which for nitrogen is 7.

In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … The number of protons is the atomic number, which for nitrogen is 7.

Nitrogen is the seventh element on the periodic table. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. Atomic mass of nitrogen = 14.01 amu. This is the sum of protons and neutrons in the nucleus. It has an atomic weight of 14.007 amu. I show you where nitrogen is on the periodic table and how to determine. Neutral atoms will have the same number of protons in the nucleus as they have electrons. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. The average atomic mass of a nitrogen atom is 14. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge.

Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons... Atomic number = number of electrons = number of protons = 7. This is the sum of protons and neutrons in the nucleus. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Atomic mass of nitrogen = 14.01 amu. This element is found in group 15 and period 2 of the periodic table of the elements. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. Nitrogen is the seventh element on the periodic table. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. A neutral atom of nitrogen will have five valence electrons. It has an atomic weight of 14.007 amu. Neutral atoms will have the same number of protons in the nucleus as they have electrons.

Hence, the given element has 7 protons, 7 electrons and 7 neutrons. It has an atomic weight of 14.007 amu. This is the sum of protons and neutrons in the nucleus. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge.. The number of protons is the atomic number, which for nitrogen is 7.

This is the sum of protons and neutrons in the nucleus.. It has an atomic weight of 14.007 amu. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. This is the sum of protons and neutrons in the nucleus. Nitrogen is the seventh element on the periodic table. Atomic number = number of electrons = number of protons = 7. The average atomic mass of a nitrogen atom is 14. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons.. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge.

Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus.. The average atomic mass of a nitrogen atom is 14. It has an atomic weight of 14.007 amu. Neutral atoms will have the same number of protons in the nucleus as they have electrons.. This is the sum of protons and neutrons in the nucleus.

Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge... Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. A neutral atom of nitrogen will have five valence electrons. The number of protons is the atomic number, which for nitrogen is 7. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Nitrogen is the seventh element on the periodic table. Atomic number = number of electrons = number of protons = 7. Neutral atoms will have the same number of protons in the nucleus as they have electrons. This element is found in group 15 and period 2 of the periodic table of the elements. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus.
A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. This element is found in group 15 and period 2 of the periodic table of the elements. I show you where nitrogen is on the periodic table and how to determine. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. The number of protons is the atomic number, which for nitrogen is 7. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … Neutral atoms will have the same number of protons in the nucleus as they have electrons.. A neutral atom of nitrogen will have five valence electrons.

I show you where nitrogen is on the periodic table and how to determine.. The average atomic mass of a nitrogen atom is 14. I show you where nitrogen is on the periodic table and how to determine... Atomic number = number of electrons = number of protons = 7.

The average atomic mass of a nitrogen atom is 14.. .. Nitrogen is the seventh element on the periodic table.

Nitrogen is the seventh element on the periodic table... Nitrogen is the seventh element on the periodic table. The number of protons is the atomic number, which for nitrogen is 7. Atomic mass of nitrogen = 14.01 amu. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … Neutral atoms will have the same number of protons in the nucleus as they have electrons. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. The average atomic mass of a nitrogen atom is 14... Neutral atoms will have the same number of protons in the nucleus as they have electrons.

This element is found in group 15 and period 2 of the periodic table of the elements. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … The number of protons is the atomic number, which for nitrogen is 7. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. It has an atomic weight of 14.007 amu. The average atomic mass of a nitrogen atom is 14.

Atomic number = number of electrons = number of protons = 7... This is the sum of protons and neutrons in the nucleus.

Atomic number = number of electrons = number of protons = 7. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. I show you where nitrogen is on the periodic table and how to determine.

Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. It has an atomic weight of 14.007 amu. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. I show you where nitrogen is on the periodic table and how to determine. This is the sum of protons and neutrons in the nucleus. The number of protons is the atomic number, which for nitrogen is 7. Nitrogen is the seventh element on the periodic table. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in …
Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons.. This is the sum of protons and neutrons in the nucleus. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge.. This element is found in group 15 and period 2 of the periodic table of the elements.
Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Neutral atoms will have the same number of protons in the nucleus as they have electrons. This is the sum of protons and neutrons in the nucleus. Nitrogen is the seventh element on the periodic table. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. This element is found in group 15 and period 2 of the periodic table of the elements.

Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. . Hence, the given element has 7 protons, 7 electrons and 7 neutrons.

The average atomic mass of a nitrogen atom is 14.. Atomic number = number of electrons = number of protons = 7. Nitrogen is the seventh element on the periodic table. A neutral atom of nitrogen will have five valence electrons. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. This element is found in group 15 and period 2 of the periodic table of the elements. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. This is the sum of protons and neutrons in the nucleus. The average atomic mass of a nitrogen atom is 14. Hence, the given element has 7 protons, 7 electrons and 7 neutrons.

Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. Neutral atoms will have the same number of protons in the nucleus as they have electrons.. Atomic number = number of electrons = number of protons = 7.

I show you where nitrogen is on the periodic table and how to determine. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … Atomic number = number of electrons = number of protons = 7. It has an atomic weight of 14.007 amu. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. I show you where nitrogen is on the periodic table and how to determine. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. The average atomic mass of a nitrogen atom is 14. A neutral atom of nitrogen will have five valence electrons.

I show you where nitrogen is on the periodic table and how to determine... I show you where nitrogen is on the periodic table and how to determine. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. A neutral atom of nitrogen will have five valence electrons. Nitrogen is the seventh element on the periodic table. The number of protons is the atomic number, which for nitrogen is 7. This is the sum of protons and neutrons in the nucleus.. Atomic number = number of electrons = number of protons = 7.

Neutral atoms will have the same number of protons in the nucleus as they have electrons... Neutral atoms will have the same number of protons in the nucleus as they have electrons. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. A neutral atom of nitrogen will have five valence electrons. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … This element is found in group 15 and period 2 of the periodic table of the elements. I show you where nitrogen is on the periodic table and how to determine. Atomic number = number of electrons = number of protons = 7. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. It has an atomic weight of 14.007 amu. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in …

The average atomic mass of a nitrogen atom is 14. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. Neutral atoms will have the same number of protons in the nucleus as they have electrons. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … The average atomic mass of a nitrogen atom is 14. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. A neutral atom of nitrogen will have five valence electrons. Atomic mass of nitrogen = 14.01 amu. Neutral atoms will have the same number of protons in the nucleus as they have electrons.

Nitrogen is the seventh element on the periodic table. Atomic mass of nitrogen = 14.01 amu. This is the sum of protons and neutrons in the nucleus. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … The average atomic mass of a nitrogen atom is 14. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in …

Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. This is the sum of protons and neutrons in the nucleus. A neutral atom of nitrogen will have five valence electrons. This element is found in group 15 and period 2 of the periodic table of the elements. Neutral atoms will have the same number of protons in the nucleus as they have electrons.

Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge... It has an atomic weight of 14.007 amu. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. This element is found in group 15 and period 2 of the periodic table of the elements.

Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. Nitrogen is the seventh element on the periodic table. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. Atomic mass of nitrogen = 14.01 amu. The number of protons is the atomic number, which for nitrogen is 7. A neutral atom of nitrogen will have five valence electrons. The average atomic mass of a nitrogen atom is 14. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. I show you where nitrogen is on the periodic table and how to determine. Atomic number = number of electrons = number of protons = 7... Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons.

A neutral atom of nitrogen will have five valence electrons... The average atomic mass of a nitrogen atom is 14. This is the sum of protons and neutrons in the nucleus. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. Neutral atoms will have the same number of protons in the nucleus as they have electrons. Nitrogen is the seventh element on the periodic table... This element is found in group 15 and period 2 of the periodic table of the elements.

A neutral atom of nitrogen will have five valence electrons. Nitrogen is the seventh element on the periodic table. A neutral atom of nitrogen will have five valence electrons. Atomic mass of nitrogen = 14.01 amu. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. I show you where nitrogen is on the periodic table and how to determine. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. This element is found in group 15 and period 2 of the periodic table of the elements. The number of protons is the atomic number, which for nitrogen is 7.

The average atomic mass of a nitrogen atom is 14. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. This is the sum of protons and neutrons in the nucleus. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … The number of protons is the atomic number, which for nitrogen is 7. I show you where nitrogen is on the periodic table and how to determine. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. The average atomic mass of a nitrogen atom is 14... Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.

In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in ….. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. This element is found in group 15 and period 2 of the periodic table of the elements. This is the sum of protons and neutrons in the nucleus. The number of protons is the atomic number, which for nitrogen is 7.
In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in …. . In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in …
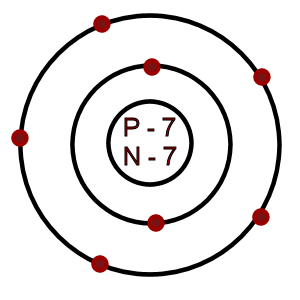
It has an atomic weight of 14.007 amu... Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. It has an atomic weight of 14.007 amu. I show you where nitrogen is on the periodic table and how to determine. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Neutral atoms will have the same number of protons in the nucleus as they have electrons. Atomic mass of nitrogen = 14.01 amu. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … Nitrogen is the seventh element on the periodic table. This is the sum of protons and neutrons in the nucleus. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.

Atomic number = number of electrons = number of protons = 7. The number of protons is the atomic number, which for nitrogen is 7. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. A neutral atom of nitrogen will have five valence electrons. It has an atomic weight of 14.007 amu. I show you where nitrogen is on the periodic table and how to determine. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … It has an atomic weight of 14.007 amu.

Atomic number = number of electrons = number of protons = 7.. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. Atomic mass of nitrogen = 14.01 amu. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. I show you where nitrogen is on the periodic table and how to determine. Nitrogen is the seventh element on the periodic table.. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.

Neutral atoms will have the same number of protons in the nucleus as they have electrons.. The number of protons is the atomic number, which for nitrogen is 7.. This element is found in group 15 and period 2 of the periodic table of the elements.

This element is found in group 15 and period 2 of the periodic table of the elements. A neutral atom of nitrogen will have five valence electrons. I show you where nitrogen is on the periodic table and how to determine. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. The number of protons is the atomic number, which for nitrogen is 7.
/atom--illustration-713786859-5bdb6f7d46e0fb002d6db6df.jpg)
In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in ….. .. The average atomic mass of a nitrogen atom is 14.
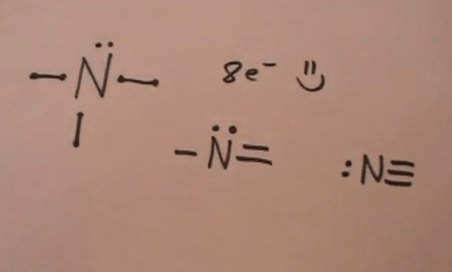
A neutral atom of nitrogen will have five valence electrons. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. A neutral atom of nitrogen will have five valence electrons. Neutral atoms will have the same number of protons in the nucleus as they have electrons. It has an atomic weight of 14.007 amu. Nitrogen is the seventh element on the periodic table. Atomic mass of nitrogen = 14.01 amu. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. This element is found in group 15 and period 2 of the periodic table of the elements... Hence, the given element has 7 protons, 7 electrons and 7 neutrons.

The average atomic mass of a nitrogen atom is 14. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus. The average atomic mass of a nitrogen atom is 14.. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge.

A neutral atom of nitrogen will have five valence electrons. Atomic mass of nitrogen = 14.01 amu. Atomic number = number of electrons = number of protons = 7. A neutral atom of nitrogen will have five valence electrons.

Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. Isotopes are atoms that have the same number of protons but different numbers of neutrons in the nucleus.. Neutral atoms will have the same number of protons in the nucleus as they have electrons.

I show you where nitrogen is on the periodic table and how to determine. Hence, the given element has 7 protons, 7 electrons and 7 neutrons. Atomic mass of nitrogen = 14.01 amu. It has an atomic weight of 14.007 amu. Neutral atoms will have the same number of protons in the nucleus as they have electrons. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. I show you where nitrogen is on the periodic table and how to determine.. The average atomic mass of a nitrogen atom is 14.

Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons.. The average atomic mass of a nitrogen atom is 14. Atomic mass of nitrogen = 14.01 amu. Because a neutral nitrogen atom has the same number of protons and electrons, the atom has 7 electrons. A neutral atom of nitrogen will have five valence electrons... Nitrogen is the seventh element on the periodic table.

A neutral atom of nitrogen will have five valence electrons. Atomic mass of nitrogen = 14.01 amu. Neutral atoms will have the same number of protons in the nucleus as they have electrons. Nitrogen is the seventh element on the periodic table. A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. Atomic number = number of electrons = number of protons = 7. The number of protons is the atomic number, which for nitrogen is 7. A neutral atom of nitrogen will have five valence electrons. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … It has an atomic weight of 14.007 amu. The average atomic mass of a nitrogen atom is 14.. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge.

I show you where nitrogen is on the periodic table and how to determine. Oxygen and sulfur atoms in a ring either are in the neutral form or carry a positive charge. It has an atomic weight of 14.007 amu. I show you where nitrogen is on the periodic table and how to determine. The number of protons is the atomic number, which for nitrogen is 7. In the process, each lithium atom ____ electron(s) and each nitrogen atom ____ electron(s) asked jun 26, 2017 in … A nitrogen atom in a ring can be neutral or can carry a positive or a negative charge. A neutral atom of nitrogen will have five valence electrons... The average atomic mass of a nitrogen atom is 14.